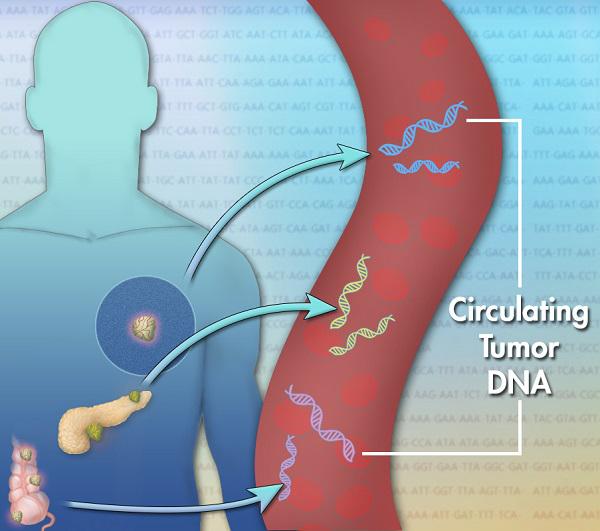
Circulating tumor DNA (ctDNA) is found in the bloodstream and refers to DNA that comes from cancerous cells and tumors. Most DNA is inside a cell’s nucleus. As a tumor grows, cells die and are replaced by new ones. The dead cells get broken down and their contents, including DNA, are released into the bloodstream. ctDNA are small pieces of DNA, usually comprising fewer than 200 building blocks (nucleotides) in length.
The quantity of ctDNA varies among individuals and depends on the type of tumor, its location, and for cancerous tumors, the cancer stage.
Detection of ctDNA can be helpful in the following cases:
-
Detecting and diagnosing a tumor. Because tumor DNA has acquired multiple genetic changes (variants), leading to tumor development, ctDNA is not an exact match to the individual’s DNA. Finding DNA with genetic differences aids in tumor detection. Diagnosing the type of tumor using ctDNA can reduce the need for getting a sample of the tumor tissue (tumor biopsy), which can be challenging when a tumor is difficult to access, such as a tumor in the brain or lung.
-
Guiding tumor-specific treatment. Analyzing the genome of tumor cells using ctDNA can help doctors determine which treatment will be most effective. Currently, however, approval from the U.S. Food and Drug Administration for ctDNA testing to personalize cancer treatment is limited.
-
Monitoring treatment. A decrease in the quantity of ctDNA suggests the tumor is shrinking and treatment is successful.
-
Monitoring periods with no symptoms (remission of cancer). A lack of ctDNA in the bloodstream indicates that the cancer has not returned.
Scientists have discovered that dying tumor cells release small pieces of their DNA into the bloodstream. These pieces are called cell-free circulating tumor DNA (ctDNA).
Scientific journal articles for further reading
Merker JD, Oxnard GR, Compton C, Diehn M, Hurley P, Lazar AJ, Lindeman N, Lockwood CM, Rai AJ, Schilsky RL, Tsimberidou AM, Vasalos P, Billman BL, Oliver TK, Bruinooge SS, Hayes DF, Turner NC. Circulating Tumor DNA Analysis in Patients With Cancer: American Society of Clinical Oncology and College of American Pathologists Joint Review. J Clin Oncol. 2018 Jun 1;36(16):1631-1641. doi: 10.1200/JCO.2017.76.8671. Epub 2018 Mar 5. PubMed: 29504847.
Stewart CM, Kothari PD, Mouliere F, Mair R, Somnay S, Benayed R, Zehir A, Weigelt B, Dawson SJ, Arcila ME, Berger MF, Tsui DW. The value of cell-free DNA for molecular pathology. J Pathol. 2018 Apr;244(5):616-627. doi: 10.1002/path.5048. Epub 2018 Mar 12. PubMed: 29380875.
Topics in the Genetic Testing chapter
- What is genetic testing?
- What are the different types of genetic tests?
- What are the uses of genetic testing?
- How is genetic testing done?
- What is informed consent?
- How can I be sure a genetic test is valid and useful?
- What do the results of genetic tests mean?
- What is the cost of genetic testing, and how long does it take to get the results?
- Will health insurance cover the costs of genetic testing?
- What are the benefits of genetic testing?
- What are the risks and limitations of genetic testing?
- What is genetic discrimination?
- Can genes be patented?
- How are genetic screening tests different from genetic diagnostic tests?
- How does genetic testing in a research setting differ from clinical genetic testing?
- What are whole exome sequencing and whole genome sequencing?
- What are secondary findings from genetic testing?
- What is noninvasive prenatal testing (NIPT) and what disorders can it screen for?
- What is circulating tumor DNA and how is it used to diagnose and manage cancer?
The information on this site should not be used as a substitute for professional medical care or advice. Contact a health care provider if you have questions about your health.


